FDA Clearance
Owlet Achieves De Novo FDA-Clearance For Dream Sock® – The First and Only Over-the-Counter, Medical Grade Pulse Oximeter Cleared for Infants
November 9, 2023
LEHI, Utah--(BUSINESS WIRE)-- Owlet, Inc. (NYSE: OWLT) (the “Company” or “Owlet”), the pioneer of smart infant monitoring, announces De Novo clearance from the U.S. Food and Drug Administration (“FDA”) of Dream Sock, the first and only over-the-counter medical pulse oximetry solution for infants. Owlet is spearheading new standards for consumer and medical solutions, allowing caregivers to provide better care at home for their babies through access to advanced digital health technologies.
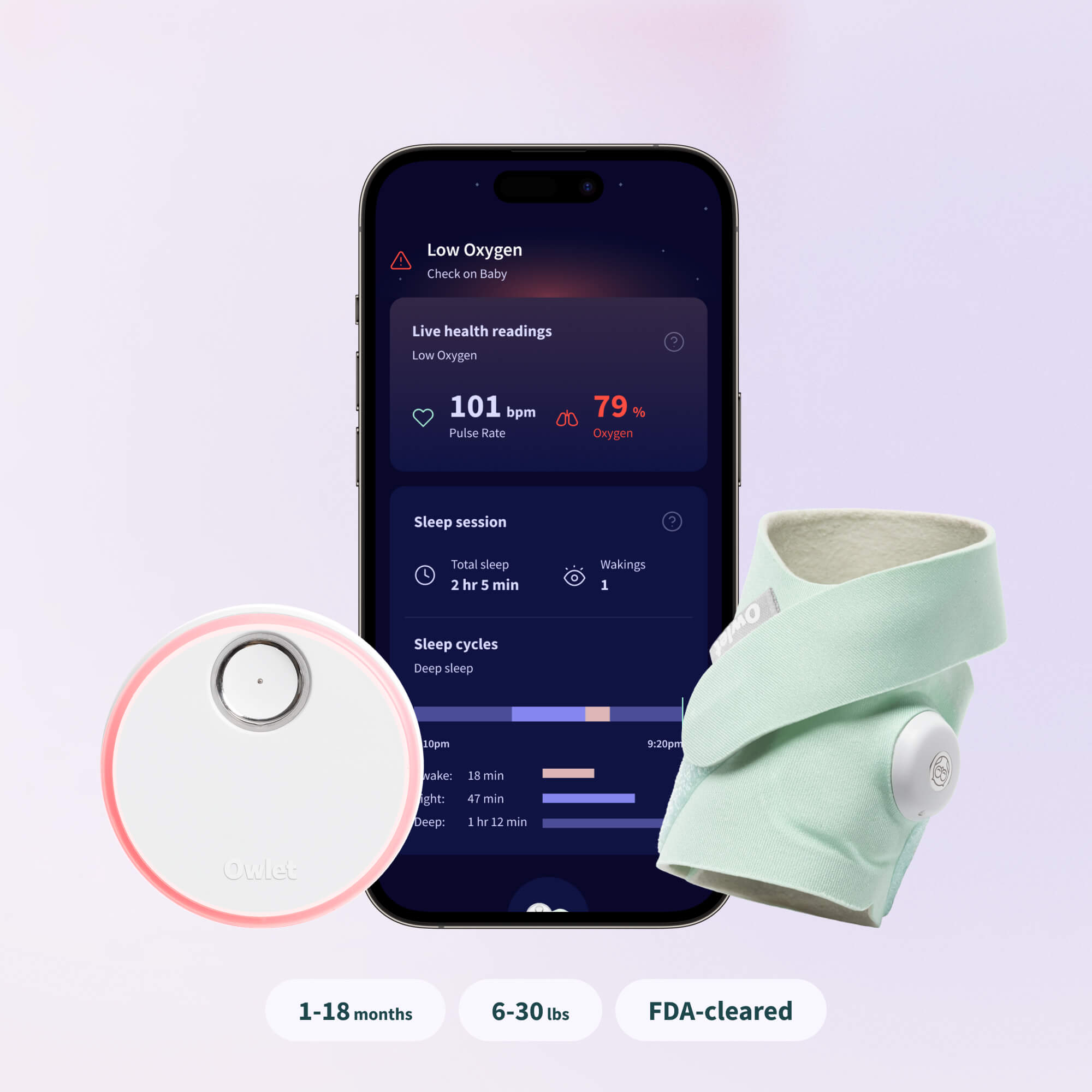
The FDA-cleared Dream Sock will monitor and display Baby’s Live Health Readings, including pulse rate and oxygen saturation level, and will provide Health Notifications, which will alert caregivers with lights and alarm sounds if their infant’s readings fall outside of preset ranges. Owlet plans to make these new medical-grade features available to all existing and new Dream Sock users upon launch, soon to be announced. These cleared features are for use with healthy infants between 1-18 months and 6 lbs to 30 lbs.
“Today marks a significant breakthrough in our journey to bring care to the home and empower parents with an unprecedented FDA-clearance for the Owlet Dream Sock,” said Kurt Workman, Owlet Chief Executive Officer and Co-Founder. “This accomplishment not only signifies our commitment to innovation in the infant health category but, more importantly, our dedication to ensuring the health and well-being of every baby. With this De Novo clearance, we are proud to set new standards in at-home infant care, arming parents with reliable real-time information and providing enhanced peace of mind.”
Achieving De Novo clearance means the Owlet Dream Sock was clinically tested in both home and hospital environments, and proven to be as accurate as medical-grade baby monitoring technology and compliant with all relevant performance and safety standards by independent laboratories. This new technology will equip caregivers with the right information at the right time, and provide them with confidence and clarity on their baby’s well-being.
“We’re all so proud that the Dream Sock was validated against the gold standards of accuracy for pulse oximetry devices – and stood up to that challenge,” said Dr. Alisa Niksch, Pediatric Cardiologist and Senior Director Medical Affairs at Owlet. “Throughout this process, we learned a tremendous amount about the capabilities of our product in supporting the care of babies in the home. We're excited to continue our research efforts as we bring new technologies and advancements in accuracy in infant monitoring to parents.”
This follows the Company’s previous FDA clearance of BabySat™, a prescription monitoring system that uses pulse oximetry technology and features a modern, wire-free sock design. Intended for infants with acute or chronic medical conditions under the supervision of a physician, BabySat provides a real-time display of a baby’s pulse rate and oxygen saturation level, and alerts parents when these customizable readings fall outside of prescribed ranges. BabySat will be available for purchase with insurance reimbursement soon. Now, Owlet’s clinically-validated technology and notification algorithms are available for use with healthy babies without a prescription.
These new FDA-cleared features will begin rolling out to both existing and new Dream Sock users by the end of this year, in the U.S. only. Parents and caregivers can learn more and sign up to receive the latest information and details on product availability at www.owletcare.com/fda-clearance.
About Owlet, Inc.
Owlet was founded by a team of parents in 2012. Owlet’s mission is to empower parents with the right information at the right time, to give them more peace of mind and help them find more joy in the journey of parenting. Owlet’s digital parenting platform aims to give parents real-time data and insights to help parents feel calmer and more confident. Owlet believes that every parent deserves peace of mind and the opportunity to feel their well-rested best. Owlet also believes that every child deserves to live a long, happy, and healthy life, and is working to develop products to help further that belief. To learn more, visit www.owletcare.com.
Owlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
LEHI, Utah — June 20, 2023 — Owlet (NYSE: OWLT, “the Company”), the pioneer of smart baby monitoring, announces clearance from the U.S. Food and Drug Administration (“FDA”) of BabySat™, Owlet's first medical pulse-oximetry device featuring its advanced, wire-free sock design. Owlet is a leader in infant health data, having monitored more than 1 million babies, and with BabySat, combines its consumer-first expertise with hospital-grade monitoring accuracy.
“Our mission is to provide caregivers with the right information at the right time to make informed decisions about their baby’s health,” said Kurt Workman, Chief Executive Officer and Co-Founder. “Today, parents whose babies need additional monitoring are sent home with traditional solutions that can be restrictive and more cumbersome for parents. BabySat pushes forward the modernization of hospital-grade technology for at-home use, and underscores our commitment to transforming baby care solutions.”
Innovation in the baby care space matters because some of the largest issues facing caregivers and healthcare providers have yet to be solved. For example, there are roughly 92 million infant care visits covering a child’s first four years of life, significantly straining the availability of hospital beds and quality of care.
BabySat is a step forward in solving some of these exact challenges by bringing real-time medical grade infant monitoring into the home, while under the supervision of a physician. Available through prescription, BabySat uses pulse oximetry technology to provide a real-time display of their baby’s pulse rate and oxygen saturation level (SpO2) and alerts parents when these readings fall outside of prescribed ranges. With access to this information and under the supervision of a physician, caregivers are enabled to feel confident in providing at-home care for their families–helping to reduce the strain on our valued medical resources.
The addition of the FDA-cleared BabySat device expands on Owlet’s existing portfolio of consumer products designed to bring peace of mind to caregivers. BabySat will be available in the U.S. only and is targeted to launch later this year. Customers can learn more and sign up to receive the latest information and details on product availability here: https://www.owletcare.com/baby...
About Owlet, Inc.
Owlet was founded by a team of parents in 2012. Owlet’s mission is to empower parents with the right information at the right time, to give them more peace of mind and help them find more joy in the journey of parenting. Owlet’s digital parenting platform aims to give parents real-time data and insights to help parents feel calmer and more confident. Owlet believes that every parent deserves peace of mind and the opportunity to feel their well-rested best. Owlet also believes that every child deserves to live a long, happy, and healthy life, and is working to develop products to help further that belief. To learn more, visit www.owletcare.com.
Media Contact:
Healthcare Contact:
Jim Fidacaro
jfidacaro@owletcare.com
Investor Contact:
Mike Cavanaugh
Westwicke/ICR
Phone: +1.617.877.9641
mike.cavanaugh@westwicke.com