Monitor What Matters Most.
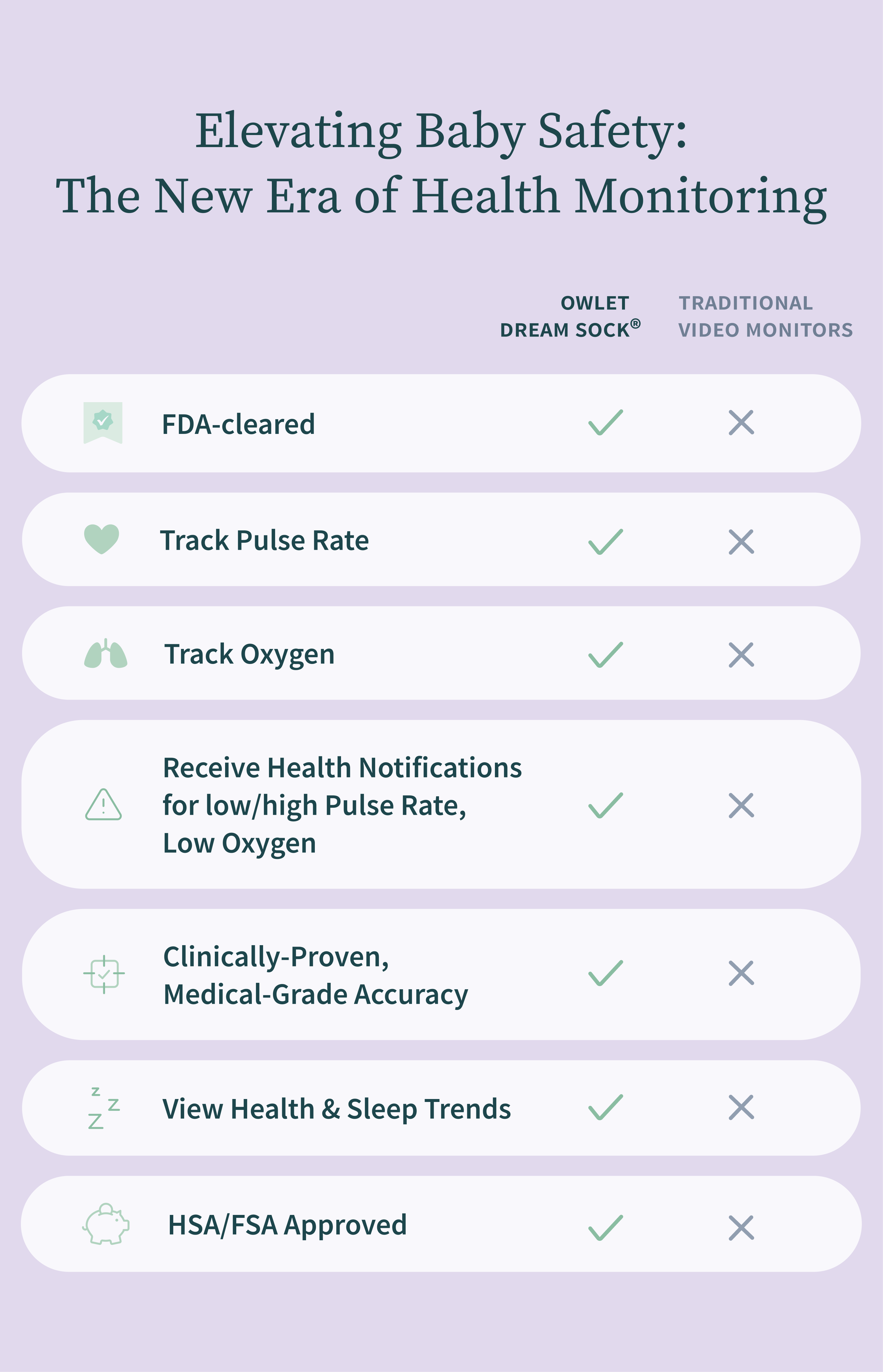
Welcome to a new era of infant care with FDA-cleared monitors that bring medical-grade monitoring to your home. View real-time health readings and receive notifications for ultimate peace of mind.

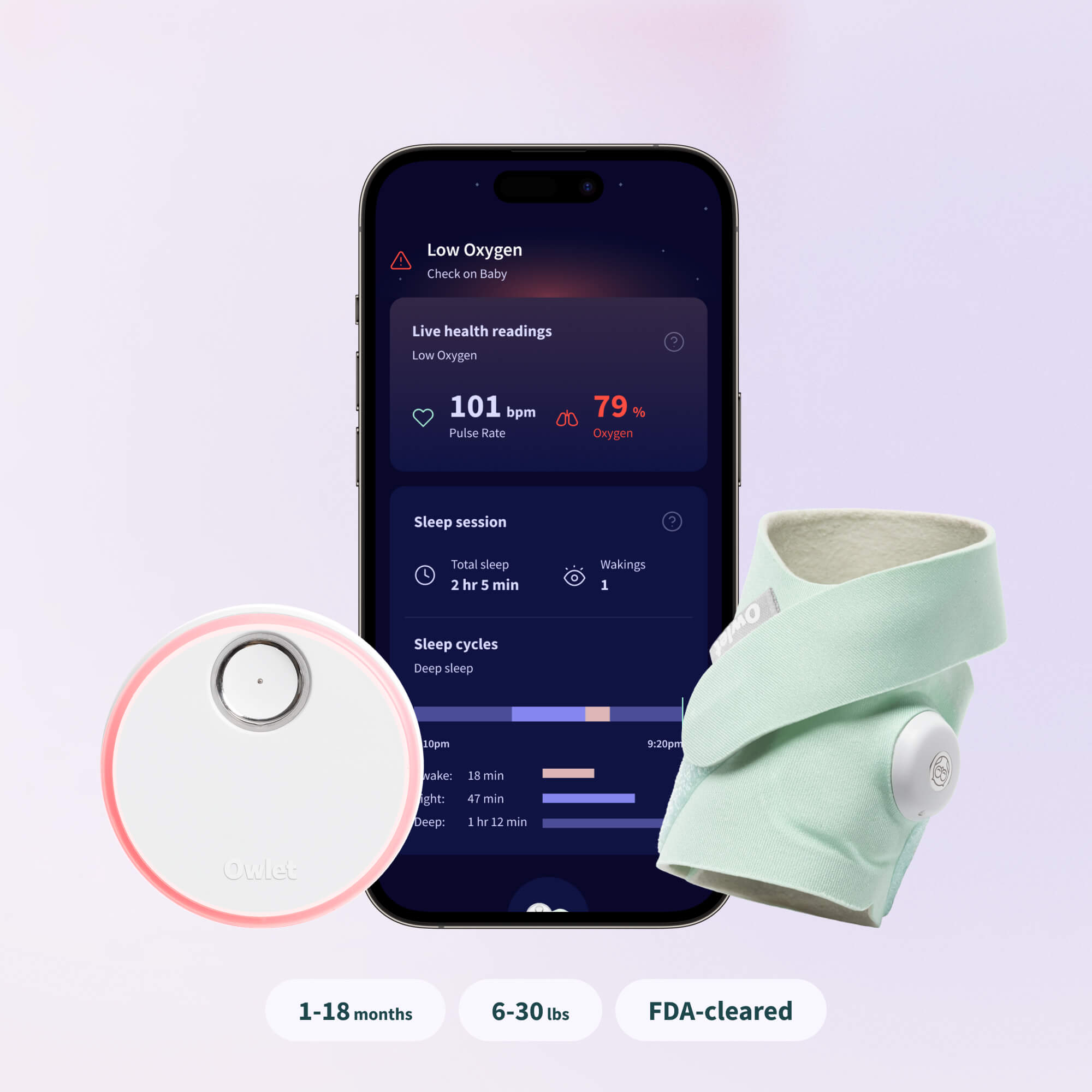
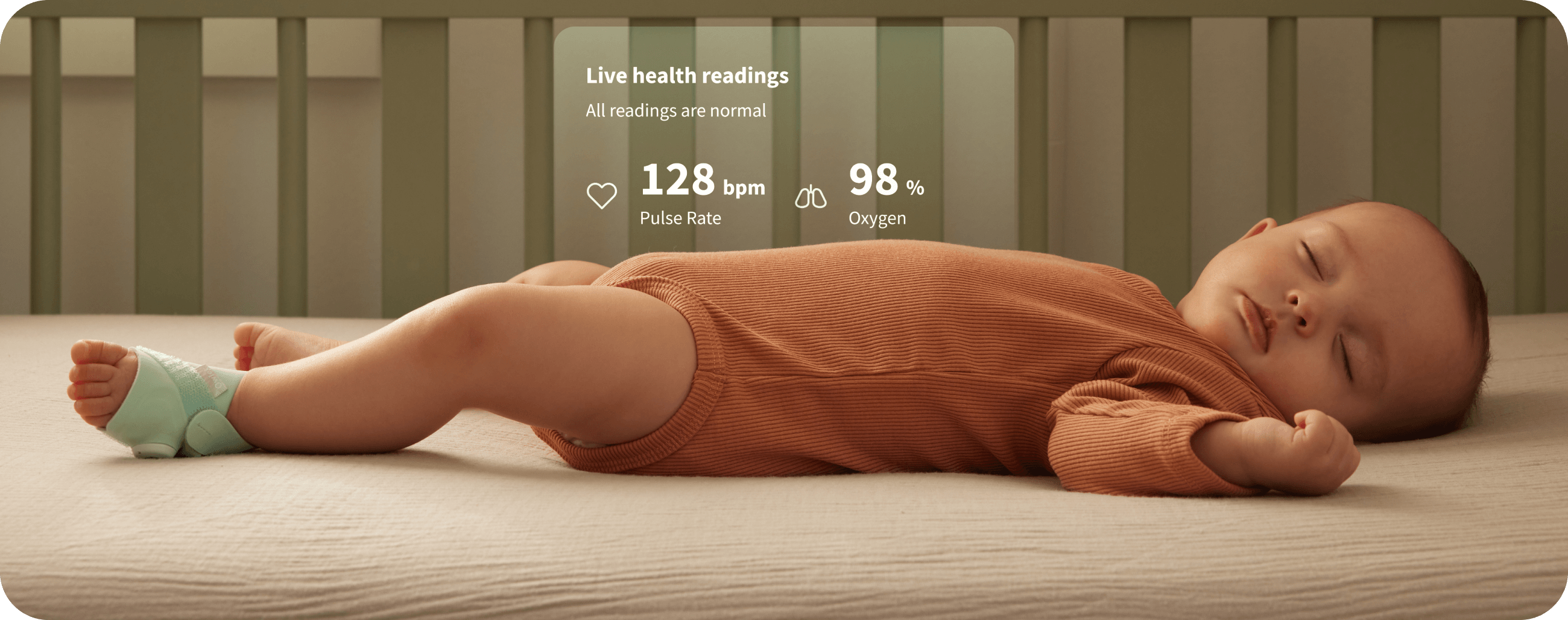
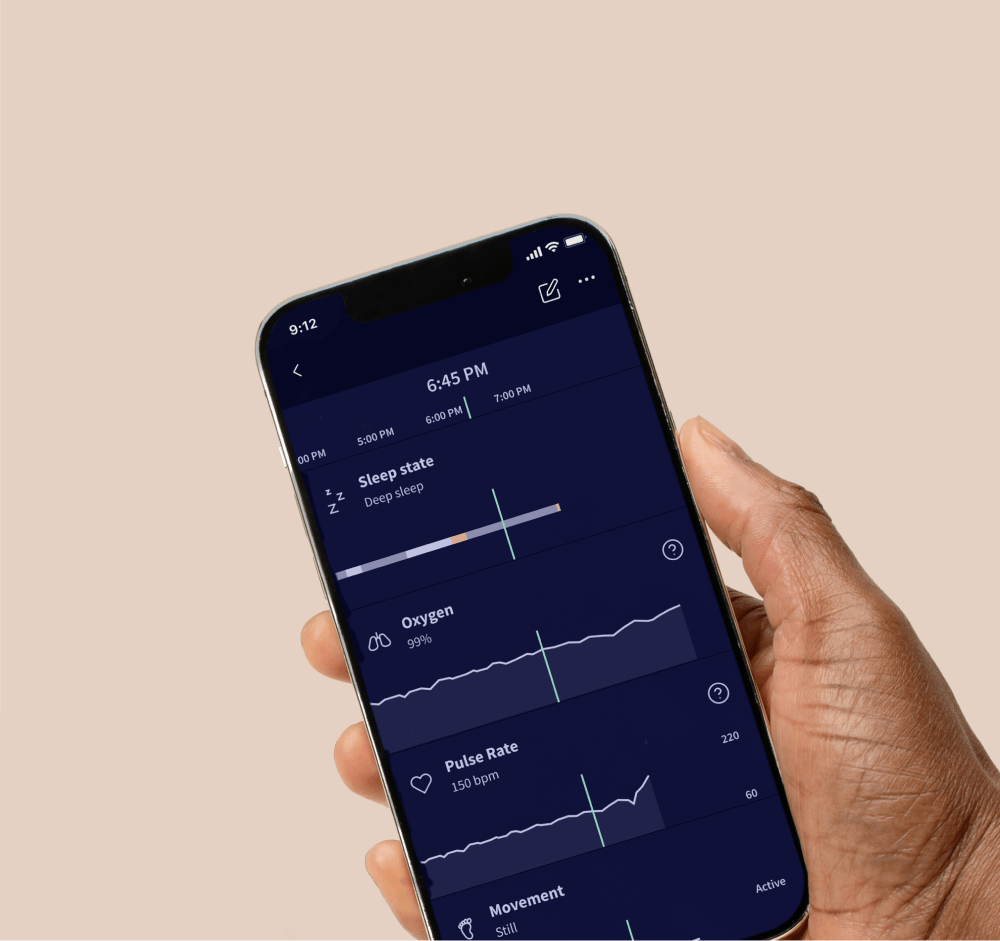
Dream Sock®
The first of its kind FDA-cleared baby monitor.

Owlet Cam 2
Our next-generation smart HD camera with night vision.

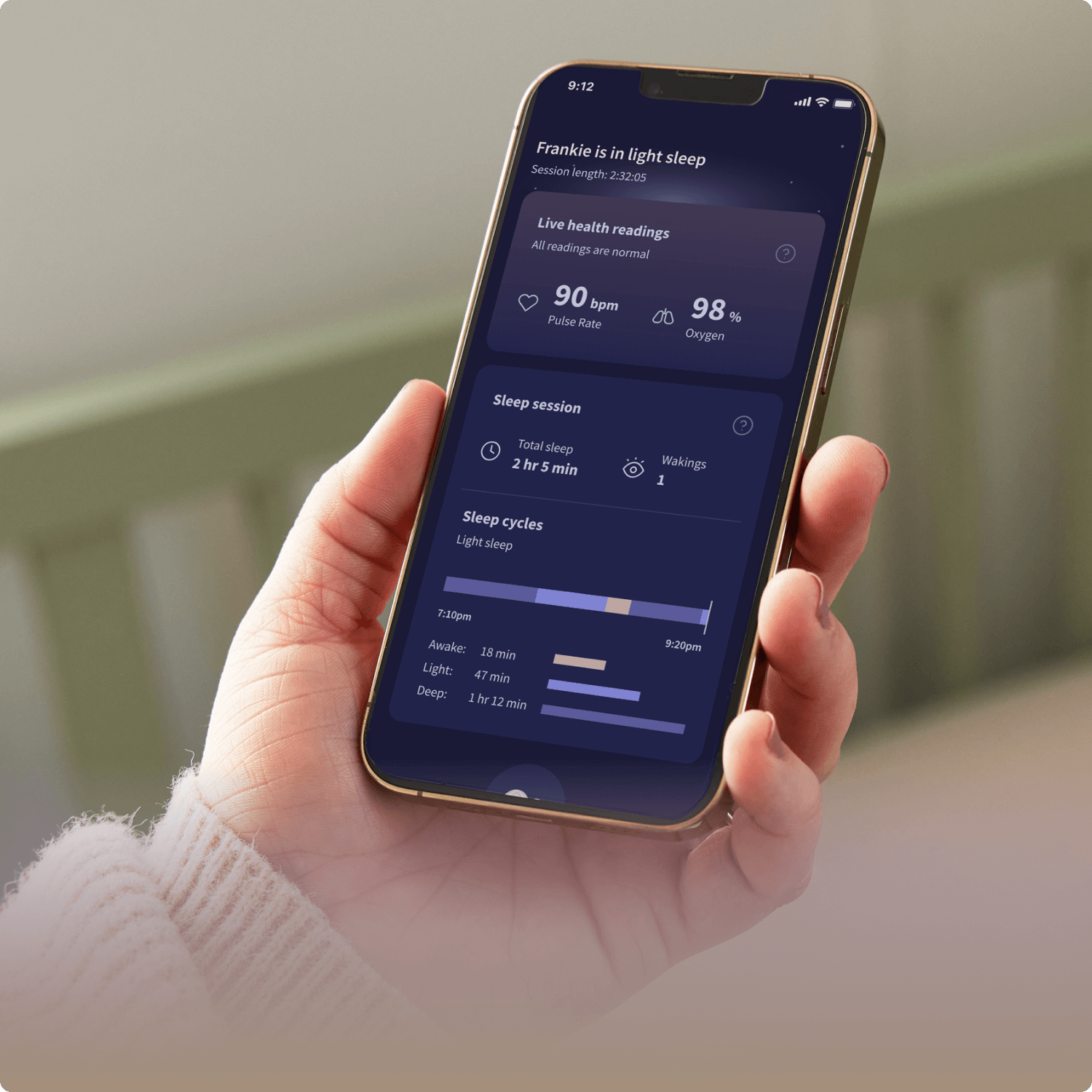
Dream Duo 2
A best seller with real-time tracking, alerts, and video.

BabySat®
Hospital-grade monitor for the home. Prescription required.
Owlet addresses one of the most significant challenges faced by parents.
Many new parents experience anxiety and concern about their baby's well-being, especially while the baby is sleeping.



STUDIES SHOW:
Parents lose an average of 133 nights' worth of sleep before their babies turn one.
STUDIES SHOW:
Sleep deprivation can worsen symptoms of postpartum depression, which affects one in eight mothers.
STUDIES SHOW:
Poor sleep is highly associated with anxiety in new mothers.
Owlet offers unparalleled peace of mind and improved sleep for families.
Our unique technology provides valuable insights that every parent needs.
DON'T JUST TAKE OUR WORD FOR IT
Life Changing Parent Stories

Lauren Fullerton (@ourfullerhome)
"So much peace of mind."
With my first two babies, I had so much anxiety about them sleeping. I was checking on them every minute and hardly sleeping myself!
With the @owletcare Dream Sock Monitor and Cam 2, I’ve received so much peace of mind. It tracks heart rate and oxygen level so I know Axel is sleeping soundly. Our bedtime routine is a success because of Owlet Care!

Kira Patton (@ki.sa)
"This Owlet Dream Duo has been life changing and I wish I had it sooner!
This Owlet Dream Duo has been life changing and I wish I had it sooner! I’m able to have a peace of mind when I go to sleep at night and not have to worry about waking up to check on my little one every hour.
The enhanced Dream Duo combines the Dream Sock and Cam 2 and is the only baby monitor to track heart rate and oxygen—all while streaming HD video right to your phone. If you are a mama, especially a first time mama I highly recommend adding this to your registry!

Tabitha Dubois
"So worth it."
After early loss in April 2021, having my son had been the best thing to ever happen to me. However, I knew my anxiety about his well-being would not subside once he was here.
I wanted the Owlet Dream Duo because I thought it would help me when he was sleeping. I knew that the dream sock displayed his heart rate and average o2 sats. And it had more than largely served its purpose. All of the other features - sleep indicators and sleep tracking- have made it that much more worth it.
We exist for every baby, every parent.
“The Owlet is like a genie when it comes to your baby’s sleep, and everyone knows there’s nothing more valuable to new parents than sleep.
“Smart baby monitors abound, but what sets the Owlet apart is the camera’s accompanying sock”

“The Best Gifts for New Moms, According to New Moms

- Independent (2018, June 19) - Referencing Simba Sleep Research
- Parents (2021, April 1} - Summarizing Snuz Sleep Survey
- Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. (2020, May 14) - Depression among women. Centers for Disease Control and Prevention.
- SleepFoundation.org (2023, June 2)