The first-of-its-kind FDA-cleared Dream Sock® smart baby monitor offers parents the ultimate peace of mind. Dream Sock provides caregivers with a deeper understanding of their infant’s well-being. Below are some frequently asked questions about our recently FDA-cleared Dream Sock.
How do I get the FDA-cleared experience? Do I need to buy a new Dream Sock?
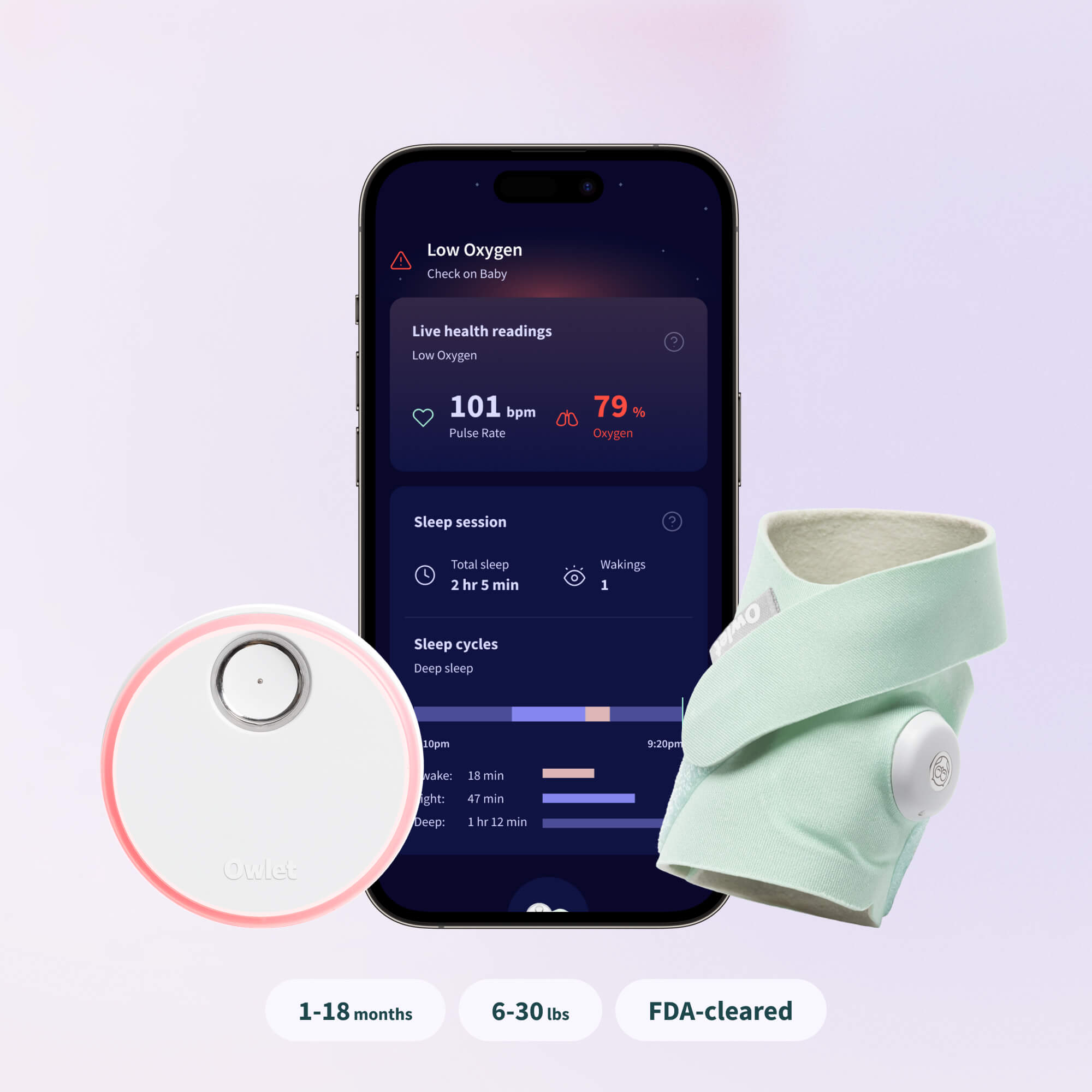
We are excited to share that you do not need to buy a new Dream Sock if you are a current user. The fully-featured FDA-cleared experience, including Live Health Readings for pulse rate and oxygen level and our new Health Notifications will be rolled out to all existing and new Dream Sock users at no extra cost.
To access FDA-cleared features, a current Dream Sock user can navigate to the Guide Tab in the Owlet Dream App and follow the upgrade instructions if their baby is between 1-18 months and 6-30 lbs.
When will the FDA-cleared Dream Sock be available?
FDA-cleared Dream Sock with Live Health Readings and Health Notifications will be rolled out very soon! We plan to begin rolling out FDA-cleared features to new and existing Dream Sock and Dream Duo users in January 2024.
What’s the difference between the previous Dream Sock experience and this new one?
The current Dream Sock experience tracks and displays live pulse rate and oxygen level, and notifies caregivers with a lavender prompt when baby may need them or their sleep quality indicators deviate from the baby’s algorithm. FDA-cleared Dream Sock will track and display Live Health Readings, including pulse rate and oxygen level. Additionally, the FDA clearance includes Owlet’s new Health Notifications, which will notify parents with lights and sounds when their child’s health readings fall outside of preset ranges.
Is Dream Sock safe and accurate?
Dream Sock is safe and accurate for meeting its intended use. Achieving this FDA De Novo clearance means Dream Sock was clinically tested and evaluated for SpO2 accuracy across all skin tones (Type I-VI on the Fitzpatrick scale) under motion and non-motion conditions. Dream Sock has been demonstrated to be accurate within +/- 3% of gold-standard arterial blood gas measurements.
Can I use Dream Sock for my child that is older than 18 months?
Dream Sock is currently intended for use on infants 0-18 months, and Dream Sock with the Extension Pack can be used up to age 5. The FDA-cleared functionality will be intended for use on infants 1-18 months and 6-30 lbs. We hope to expand this age range in the future and will share updates as we have them.
Is Dream Sock covered by insurance?
At the time of availability, Dream Sock will not be covered by insurance but is HSA/FSA eligible. There will be insurance reimbursement options available for BabySat™, our prescription monitor intended for infants that require additional monitoring under the supervision of a medical provider.
What is the difference between Dream Sock and BabySat™?
Dream Sock is the first and only FDA-cleared over-the-counter pulse oximeter for healthy infants. It is intended for use on healthy infants 1-18 months and 6-30 lbs. It features Live Health Readings, including pulse rate and oxygen level and Health Notifications that will notify caregivers with lights and sounds if levels leave preset ranges. Dream Sock pairs with the Owlet Dream App and does not qualify for insurance reimbursement. However, it is HSA/FSA eligible.
BabySat is an FDA-cleared prescription pulse oximeter that is intended for use on infants that require additional monitoring under the supervision of a medical provider. Caregivers receive alerts if levels leave ranges set by the infant’s physician. BabySat pairs with the Owlet Care+ App and does qualify for insurance reimbursement options.
FDA-cleared Dream Sock functionality will be available by January 2024 in the US. Sign up for the latest updates here.